5′-Vitamin B12 derivatives suitable for bioconjugation via the amide bond†
Abstract
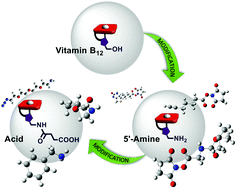
Vitamin B12 is an attractive candidate for a drug or an imaging-agent carrier into cells, due to its dietary uptake and well established transport through glycoproteins. Utilization of this system requires an appropriate functionalization of vitamin B12 that both allows for the conjugation of therapeutics and does not interrupt its recognition by transport proteins. Modifications at the 5′-position on the ribose moiety are among a few approaches which meet the criteria. In this article we present vitamin B12 derivatives bearing either the amino or the carboxylic group at the 5′-position. The presence of these functional groups enables conjugation of biologically important molecules to vitamin B12via the amide bond. The established method is not only limited to organic media but also works in an aqueous environment, giving the desired products in very good yields.


 Please wait while we load your content...
Please wait while we load your content...