Iodine(iii)-mediated intramolecular sulfeno- and selenofunctionalization of β,γ-unsaturated tosyl hydrazones and oximes†
Abstract
A cascade radical cyclization/sulfenylation or selenylation of β,γ-unsaturated hydrazones and oximes was realized under mild conditions with phenyliodine(III) diacetate (PIDA) as the sole oxidant, leading to the construction of diversely functionalized heteroatom-containing pyrazoline and isoxazoline derivatives. This metal-free radical process is suggested to encompass a sequential C–N/O and C–S/Se bond fomation.
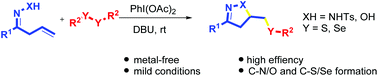


 Please wait while we load your content...
Please wait while we load your content...