Transamidation of N-acyl-glutarimides with amines†
Abstract
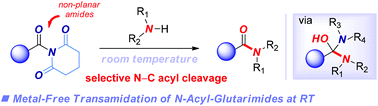
The development of new transamidation reactions for the synthesis of amides is an important and active area of research due to the central role of amide linkage in various fields of chemistry. Herein, we report a new method for transamidation of N-acyl-glutarimides with amines under mild, metal-free conditions that relies on amide bond twist to weaken amidic resonance. A wide range of amines and functional groups, including electrophilic substituents that would be problematic in metal-catalyzed protocols, are tolerated under the reaction conditions. Mechanistic experiments implicate the amide bond twist, thermodynamic stability of the tetrahedral intermediate and leaving group ability of glutarimide as factors controlling the reactivity of this process. The method further establishes the synthetic utility of N-acyl-glutarimides as bench-stable, twist-perpendicular, amide-based reagents in acyl-transfer reactions by a metal-free pathway. The origin of reactivity of N-acyl-glutarimides in metal-free and metal-catalyzed processes is discussed and compared.


 Please wait while we load your content...
Please wait while we load your content...