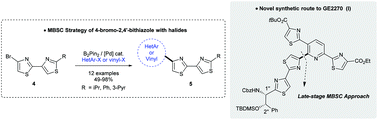
Miyaura borylation/Suzuki–Miyaura coupling (MBSC) sequence of 4-bromo-2,4′-bithiazoles with halides: straightforward access to a heterocylic cluster of d-series of thiopeptide GE2270†
Abstract
Herein, palladium-catalyzed Miyaura borylation of 4-bromo-2,4′-bithiazoles followed by Suzuki–Miyaura cross-coupling reaction (named the MBSC process) with (hetero)aryl- and alkenyl halides is reported. This methodology offers rapid access to various 2′,4-disubstituted 2,4′-bithiazole features including naturally-occurring 4-alkenylated and 4-pyridinylated 2,4′-bithiazoles. To prove its application, a concise approach for the synthesis of a heterocyclic cluster of the thiopeptide D-series antibiotic GE2270 is reported through a late-stage MBSC strategy.


 Please wait while we load your content...
Please wait while we load your content...