Convenient, functional group-tolerant, transition metal-free synthesis of aryl and heteroaryl trifluoromethyl ketones with the use of methyl trifluoroacetate†
Abstract
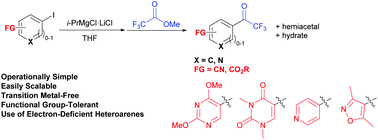
A new convenient, functional group-tolerant, transition metal-free route to aryl trifluoromethyl ketones under mild conditions is described. The reaction of not only aryl Grignard reagents carrying reducible electrophilic functional groups, such as ester and cyano groups, but also electron-deficient nitrogen-containing heteroaryl Grignard reagents with methyl trifluoroacetate gives the corresponding aryl or heteroaryl trifluoromethyl ketones in good yields. Biological assays revealed that new heteroaryl trifluoromethyl ketones carrying 2,4-dimethoxypyrimidine and 3,5-dimethylisoxazole rings show good anti-tumor activities.


 Please wait while we load your content...
Please wait while we load your content...