DABCO-catalyzed silver-promoted direct thiolation of pyrazolones with diaryl disulfides†
Abstract
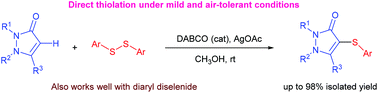
A highly efficient protocol for a direct thiolation of N-substituted pyrazolones with diaryl disulfides is described. Using a combination of DABCO and silver(I) acetate, the C–S bond formation proceeds smoothly at room temperature under mild and easy to handle conditions. This synthetic strategy offers a convenient and direct modification of antipyrine and other pyrazolone substrates, giving a series of aryl sulfide-substituted pyrazolone products in moderate to excellent yields.


 Please wait while we load your content...
Please wait while we load your content...