Bioinspired total synthesis of tetrahydrofuran lignans by tandem nucleophilic addition/redox isomerization/oxidative coupling and cycloetherification reactions as key steps†
Abstract
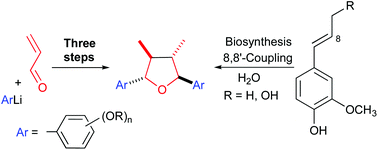
A very short three-step approach to trans,trans,trans-2,5-diaryl-3,4-dimethyltetrahydrofuran lignans is reported. The carbon skeleton is assembled in a single step based on an unprecedented tandem reaction consisting of 1,2-addition of aryllithium reagents to α,β-unsaturated aldehydes, ruthenium-catalyzed redox isomerization of the resulting alkoxides to enolates and their dimerization triggered by single electron oxidation. The resulting 2,3-dialkyl-1,4-diketones form with moderate to good d/l-diastereoselectivity and are transformed to the target tetrahydrofuran lignans by reduction and diastereoselective cycloetherification.


 Please wait while we load your content...
Please wait while we load your content...