Arginine-selective bioconjugation with 4-azidophenyl glyoxal: application to the single and dual functionalisation of native antibodies†
Abstract
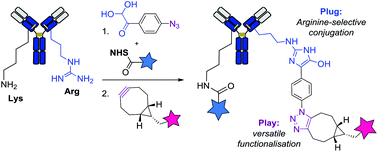
Here, we introduce 4-azidophenyl glyoxal (APG) as an efficient plug-and-play reagent for the selective functionalisation of arginine residues in native antibodies. The selective reaction between APG and arginines’ guanidine groups allowed a facile introduction of azide groups on the monoclonal antibody trastuzumab (plug stage). These pre-functionalised antibody–azide conjugates were then derivatised during the “play stage” via a biorthogonal cycloaddition reaction with different strained alkynes. This afforded antibody-fluorophore and antibody–oligonucleotide conjugates, all showing preserved antigen selectivity and high stability in human plasma. Due to a lower content of arginines compared to lysines in native antibodies, this approach is thus attractive for the preparation of more homogeneous conjugates. This method proved to be orthogonal to classical lysine-based conjugation and allowed straightforward generation of dual-payload antibody.


 Please wait while we load your content...
Please wait while we load your content...