Mannich reaction of trifluoroacetaldehyde hydrazones: a useful entry to trifluoromethyl substituted heterocycles†
Abstract
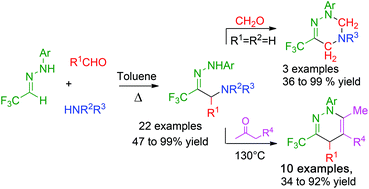
NH-aryl hydrazones derived from trifluoroacetaldehyde hemiacetal may be involved in efficient Mannich type reactions with formaldehydes and aromatic aldehydes. The resulting hydrazones are useful building blocks for the preparation of trifluoromethyl substituted heterocycles as shown by a straightforward preparation of 1,2-diazine derivatives under heating with β-ketoesters.


 Please wait while we load your content...
Please wait while we load your content...