Discovery of novel diarylpyrimidines as potent HIV-1 NNRTIs by investigating the chemical space of a less explored “hydrophobic channel”
Abstract
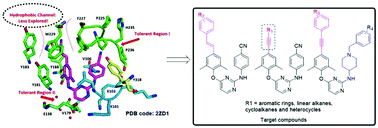
A new series of diarylpyrimidines (DAPYs) were designed, synthesized and evaluated as novel HIV-1 NNRTIs to further explore the chemical space surrounding the “hydrophobic channel” of the NNRTI binding pocket (NNIBP), guided by the comprehensive analysis of X-ray structural biology data of HIV-1 RT/NNRTI complexes and molecular modeling. Encouragingly, most of the synthesized DAPYs were found to be active against the HIV-1 wild-type (WT) strain with EC50 values ranging from 3 nM to 63 nM, and displayed significantly reduced cytotoxicity compared with etravirine (ETV) and rilpivirine (RPV). Among them, two most promising compounds Z10 (EC50 = 3 nM) and Z13 (EC50 = 3 nM) showed equivalent potency against the HIV-1 WT strain to the reference drugs efavirenz (EFV, EC50 = 3 nM) and ETV (EC50 = 3 nM). Notably, Z13 also showed the most potent activity against HIV-1 mutant strains including K103N (EC50 = 10 nM), E138K (EC50 = 22 nM) and RES056 (EC50 = 0.935 μM). Against mutant strains Y181C, Y188L and F227L + V106A, Z17 showed double-digit nanomolar inhibitory activity with EC50 values 27 nM, 98 nM and 30 nM, respectively. The structure–activity relationships (SARs) and molecular docking studies provided important clues for further molecular elaboration. Collectively, this study provides useful information to guide lead optimization and drug discovery via the exploration of this seldom investigated region.


 Please wait while we load your content...
Please wait while we load your content...