Synthesis of spiro-4H-pyrazole-oxindoles and fused 1H-pyrazoles via divergent, thermally induced tandem cyclization/migration of alkyne-tethered diazo compounds†
Abstract
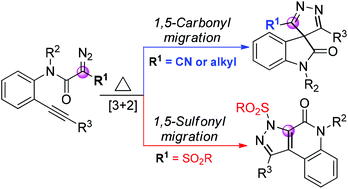
A thermally induced, substrate-dependent reaction of alkynyl diazo compounds has been developed. This transformation produces spiro-4H-pyrazole-oxindoles and fused 1H-pyrazoles in good to high yields from the corresponding alpha-cyano and alpha-sulfonyl diazo compounds. The salient features of this reaction include excellent chemoselectivity and atom-economy, mild reaction conditions, simple purification and potential for large scale production.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...