Governing the DNA-binding mode of styryl dyes by the length of their alkyl substituents – from intercalation to major groove binding†
Abstract
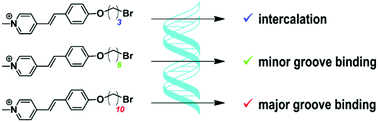
A series of monomeric and homodimeric 4-alkoxystyryl(pyridinium) dyes was synthesized and their DNA-binding properties were investigated. We found that the length of the alkyl substituent has a crucial influence on the binding mode of the dyes, although the structure of the DNA-binding unit is the same for all compounds. Remarkably, mono- and bis-styryl derivatives comprising an oxodecyl chain represent the rare examples of small molecules that bind to the major groove of DNA. We have also demonstrated that the dyes, except the monostyryl dye with a bromopropyl substituent, form chiral aggregates in the presence of double-stranded DNA.


 Please wait while we load your content...
Please wait while we load your content...