A domino reaction of 3-chlorochromones with aminoheterocycles. Synthesis of pyrazolopyridines and benzofuropyridines and their optical and ecto-5′-nucleotidase inhibitory effects†
Abstract
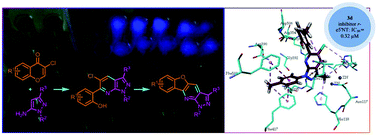
A new and efficient domino reaction of 3-chlorochromones with electron-rich aminoheterocycles was developed which allows for a convenient synthesis of a variety of pyrazolo[3,4-b]pyridines, pyrrolo[2,3-b]pyridines, pyrido[2,3-d]pyrimidines and benzofuro[3,2-b]pyridines. The products exhibit strong fluorescence. In addition, they exhibit significant ecto-5′-nucleotidase inhibition properties and cytotoxic behavior.


 Please wait while we load your content...
Please wait while we load your content...