Synthesis of spiro-tetrahydrothiopyran-oxindoles by Michael–aldol cascade reactions: discovery of potential P53-MDM2 inhibitors with good antitumor activity†
Abstract
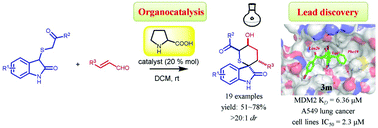
Using proline as the catalyst, an organocatalytic Michael–aldol cascade reaction was developed for the synthesis of spiro-tetrahydrothiopyran oxindoles. The highly functionalized scaffold was assembled in moderate to good yields (51–78%) and excellent diastereoselectivities (>20 : 1 dr). Interestingly, the oxindoles displayed moderate to good in vitro antitumor activities and were validated as p53-MDM2 inhibitors, which represented promising lead compounds for antitumor drug discovery.


 Please wait while we load your content...
Please wait while we load your content...