Catalytic enantioselective synthesis of carboxy-substituted 2-isoxazolines by cascade oxa-Michael-cyclization†
Abstract
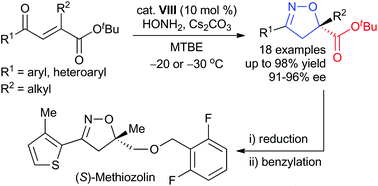
An efficient quinidine-based phase-transfer-catalyzed enantioselective cascade oxa-Michael-cyclization reaction of hydroxylamine with various β-carboxy-substituted α,β-unsaturated ketones has been achieved for the preparation of chiral carboxy-substituted 2-isoxazolines. This cascade reaction provided the desired products in good yields (up to 98%) with excellent enantioselectivities (91–96% ee). In addition, the cascade reaction was effectively applied to the first catalytic asymmetric synthesis of the herbicide (S)-methiozolin.


 Please wait while we load your content...
Please wait while we load your content...