A dipeptide with enhanced anion binding affinity enables cell uptake and protein delivery†
Abstract
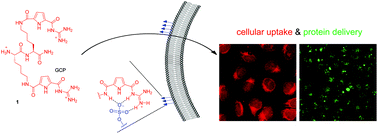
Herein, we report a rather simple strategy to enhance the anion binding ability of a dipeptide to achieve cell uptake and also protein delivery. Peptide 1, composed of only two synthetic amino acids with an artificial anion binding site in the side chains, has an overall molecular weight of only 630 Da and demonstrated strong binding affinity (107 M−1) and clustering ability with heparin as a model for cell surface sugars. Furthermore, peptide 1 is also efficiently taken up by cells most likely via endocytosis. The uptake efficiency is dependent on the amount of glycosaminoglycans on the cell surface. Cells with reduced amounts of surface bound glycosaminoglycans show significantly less uptake of peptide 1. Moreover, 1 induced the uptake of a model protein (avidin, around 67 kDa) into cells, which makes 1 a highly attractive candidate for drug and protein delivery, especially as 1 has negligible cytotoxicity.

 Please wait while we load your content...
Please wait while we load your content...