The direct and highly diastereoselective synthesis of 3,4-epoxy-2-piperidones. Application to the total synthesis and absolute configurational assignment of 3α,4α-epoxy-5β-pipermethystine†
Abstract
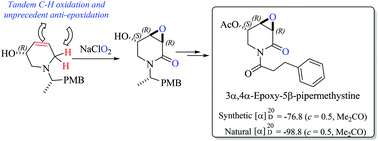
The substrate-controlled asymmetric total synthesis and absolute configurational assignment of biologically active 3α,4α-epoxy-5β-pipermethystine, a minor component in the aerial parts of kava, has been achieved by featuring, as a key step, the environmentally friendly and direct synthesis of 2,3-epoxyamides from allyl amines. By using the chiron approach, first a carbohydrate-derived dehydropiperidine was prepared and subjected to a stereoselective tandem C–H/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C oxidation reaction. In this attempt, the required α,α-trans-epoxy-2-piperidone skeleton of the kava metabolite precursor was not achieved, although the tandem oxidation was highly stereoselective. However, starting from non-carbohydrate 3-hydroxy-4,5-dehydropiperidine, and using the same tandem oxidation, the target intermediate was obtained in high yield and complete unprecedented anti-stereoselectivity. Since the proposed mechanistic course of this tandem oxidation implies the transient formation of an α,β-unsaturated amide followed by the subsequent epoxidation reaction, this second approach supports the previously established biotransformation proposal of (−)-pipermethystine to (−)-3α,4α-epoxy-5β-pipermethystine.
C oxidation reaction. In this attempt, the required α,α-trans-epoxy-2-piperidone skeleton of the kava metabolite precursor was not achieved, although the tandem oxidation was highly stereoselective. However, starting from non-carbohydrate 3-hydroxy-4,5-dehydropiperidine, and using the same tandem oxidation, the target intermediate was obtained in high yield and complete unprecedented anti-stereoselectivity. Since the proposed mechanistic course of this tandem oxidation implies the transient formation of an α,β-unsaturated amide followed by the subsequent epoxidation reaction, this second approach supports the previously established biotransformation proposal of (−)-pipermethystine to (−)-3α,4α-epoxy-5β-pipermethystine.


 Please wait while we load your content...
Please wait while we load your content...