Catalytic asymmetric construction of spiropyrrolidines via 1,3-dipolar cycloaddition of azomethine ylides
Abstract
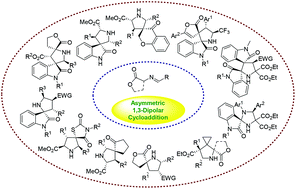
Spirocyclic pyrrolidines are structural motifs frequently found in a wide variety of natural products, pharmaceuticals and biologically significant compounds. In the past few years, catalytic asymmetric 1,3-dipolar cycloaddition reactions of azomethine ylides have shown to be one of the most straightforward methods for the stereoselective preparation of diverse biologically important spiropyrrolidine heterocycles in high yields with excellent enantioselectivities under mild reaction conditions. In this review, we will discuss the recent major developments in the catalytic enantioselective synthesis of chiral spiropyrrolidine derivatives since 2009.


 Please wait while we load your content...
Please wait while we load your content...