Design, synthesis, and conformational analysis of 3-cyclo-butylcarbamoyl hydantoins as novel hydrogen bond driven universal peptidomimetics†
Abstract
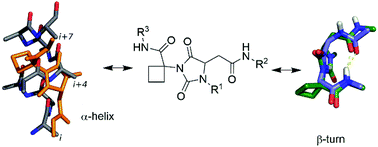
A collection of systematically substituted 3-cyclo-butylcarbamoyl hydantoins was synthesized by a regioselective multicomponent domino process followed by easy coupling reactions. Calculations, NMR studies and X-ray analysis show that these scaffolds are able to project their side chains similar to common secondary structures, such as the α-helix and β-turn, with favourable enthalpic and entropic profiles.


 Please wait while we load your content...
Please wait while we load your content...