A Brønsted acid-promoted asymmetric intramolecular allylic amination of alcohols†
Abstract
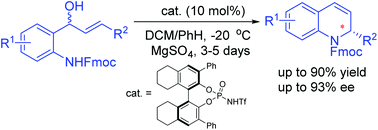
Reported herein is a chiral Brønsted acid-catalyzed asymmetric intramolecular allylic amination reaction, allowing facile access to a range of biologically interesting chiral 2-substituted hydroquinolines in up to 90% yield and with up to 93% ee. Furthermore, a significant effect of an N-protecting group was observed in this asymmetric process.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...