A BF3·Et2O catalyzed atom-economical approach to highly substituted indole-3-carbinols from nitrosobenzenes and propargylic alcohols†‡
Abstract
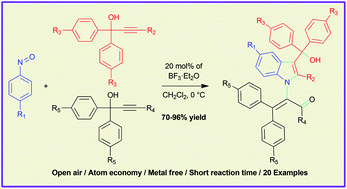
The BF3·Et2O catalyzed reaction of nitrosobenzenes and an excess amount of propargylic alcohols was investigated to synthesize highly-substituted indole-3-carbinols. This reaction involves formal [3 + 2]-cycloaddition and the subsequent 1,3-rearrangement in a tandem manner via 3-alkylidene-3H-indole N-oxides. This methodology involves the sequential addition of propargylic alcohols and inexpensive Lewis acid catalyst, occurs in an open-air environment and is atom economical. The highly-substituted indole-3-carbinols were obtained in short time and the operational simplicity allows for a large scale experiment.


 Please wait while we load your content...
Please wait while we load your content...