Synthesis of polysubstituted pyridines from oxime acetates using NH4I as a dual-function promoter†
Abstract
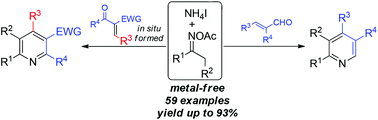
Pyridine formation with oxime acetates as the building blocks under metal-free conditions is described. Ammonium iodide has proved to be a highly efficient promoter for oxime N–O bond reduction and subsequent condensation reactions, whereby it played a dual-function role in the transformation. While the three-component reaction of oxime acetates, benzaldehydes, and 1,3-dicarbonyls proceeded well with the assistance of a stoichiometric amount of ammonium iodide, the condensation of oximes and acroleins was enabled by using a catalytic initiator to afford substituted pyridines. By this protocol, substituted pyridine products were generated in moderate to excellent yields with tolerance towards a broad range of functional groups.


 Please wait while we load your content...
Please wait while we load your content...