Rh(iii)-Catalyzed ortho-C–H alkynylation of N-phenoxyacetamides with hypervalent iodine-alkyne reagents at room temperature†
Abstract
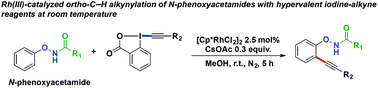
A Rh(III)-catalyzed C–H alkynylation of substituted N-phenoxyacetamides has been developed with the aid of hypervalent iodine-alkyne reagents. Complementary to the Sonogashira coupling reaction, this protocol provides an efficient and straightforward method to access aryl alkynes at room temperature. The multifunctional directing group is preserved which can be further employed for ortho-directed functionalizations to obtain additional new complex products.
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...