Ligand-free, palladacycle-facilitated Suzuki coupling of hindered 2-arylbenzothiazole derivatives yields potent and selective COX-2 inhibitors†
Abstract
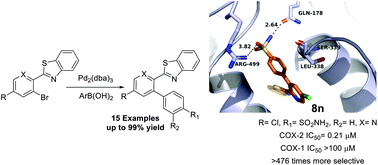
A similarity search and molecular modeling study suggested the 2′-aryl-2-arylbenzothiazole framework as a novel scaffold for the design of COX-2-selective inhibitors. Conventional Suzuki coupling conditions did not furnish the designed compounds in good yield from 2′-bromo-2-arylbenzothiazole as the starting material. A novel ligand-free Suzuki–Miyaura coupling methodology was developed for sterically hindered 2′-bromo-2-arylbenzothiazoles. The reaction depends on the coordination properties of the benzothiazole ring nitrogen, which is involved in the formation of a palladacyclic intermediate that was synthesized independently and converted to the final product. The new method provides good to excellent yields (up to 99%) with favorable functional group tolerability. Six compounds had potencies in the submicromolar range against COX-2 and higher selectivity for COX-2 vs. COX-1 compared to the currently used drug celecoxib. Molecular modeling was used to investigate the possible binding mode with COX-2.


 Please wait while we load your content...
Please wait while we load your content...