Anti-HIV activity of new higher order G-quadruplex aptamers obtained from tetra-end-linked oligonucleotides†
Abstract
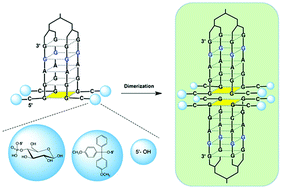
By combining the ability of short G-rich oligodeoxyribonucleotides (ODNs) containing the sequence 5′CGGA3′ to form higher order G-quadruplex (G4) complexes with the tetra-end-linked (TEL) concept to produce aptamers targeting the HIV envelope glycoprotein 120 (gp120), three new TEL-ODNs (1–3) having the sequence 5′CGGAGG3′ were synthesized with the aim of studying the effect of G4 dimerization on their anti-HIV activity. Furthermore, in order to investigate the effect of the groups at the 5′ position, the 5′ ends of 1–3 were left uncapped (1) or capped with either the lipophilic dimethoxytrityl (DMT) (2) or the hydrophilic glucosyl-4-phosphate (3) moieties. The here reported results demonstrate that only the DMT-substituted TEL-ODN 2 is effective in protecting human MT-4 cell cultures from HIV infection (76% max protection), notwithstanding all the three new aptamers proved to be capable of forming stable higher order dimeric G4s when annealed in K+-containing buffer, thus suggesting that the recognition of a hydrophobic pocket on the target glycoprotein by the aptamers represents a main structural feature for triggering their anti-HIV activity.
- This article is part of the themed collection: Chemical Biology in OBC

 Please wait while we load your content...
Please wait while we load your content...