Revisiting graphene–polymer nanocomposite for enhancing anticorrosion performance: a new insight into interface chemistry and diffusion model†
Abstract
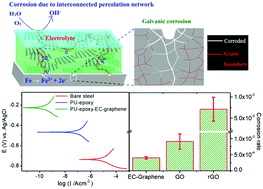
Graphene is impermeable to all molecules and has high chemical stability, which makes it an excellent anticorrosion coating for metals. However, current studies have indicated that galvanic coupling between graphene and a metal actually accelerates corrosion at the interface. Due to the insulating nature of polymers, graphene–polymer composite coatings with a strong interaction between the filler and the polymer matrix are an alternative means of addressing this issue. Nevertheless, such coatings require well-dispersed graphene flakes to lengthen the diffusion paths of gases or liquids, while preventing the formation of a conducting network from graphene to the metal. The difficulty in preparing such coatings was mainly due to problems with the control of the assembled phase during interfacial reactions. Herein, the interactions between the filler and the polymer were found to be a key factor governing anticorrosion performance, which has scarcely been previously reported. The advantage of graphene as a filler in anticorrosion coatings lies in its dispersibility and miscibility with both the casting solvent and the polymer. Electrochemically exfoliated graphene (EC-graphene) with appropriate surface functionalities that allow high miscibility with waterborne polyurethane (PU) and hydrophobic epoxy has been found to be an ideal filler that outperforms other graphene materials such as graphene oxide (GO) and reduced graphene oxide (rGO). Furthermore, a bilayer coating with EC-graphene additives for PU over epoxy has been found to reduce the corrosion rate (CR) to 1.81 × 10−5 mm per year. With a graphene loading of less than 1%, this represents the lowest CR ever achieved for copper and steel substrates and a diffusion coefficient that is lower by a factor of nearly 2.2 than that of the pristine polymer. Furthermore, we have shown that by controlling the amount of graphene loaded in the polymer galvanic corrosion favored by the formation of an interconnected graphene percolation network can successfully be limited. The present study, together with a facile and eco-friendly method of nanocomposite synthesis, may pave the way toward practical applications in the development of graphene-based anticorrosion coatings.


 Please wait while we load your content...
Please wait while we load your content...