Phase-pure pentlandite Ni4.3Co4.7S8 binary sulfide as an efficient bifunctional electrocatalyst for oxygen evolution and hydrogen evolution†
Abstract
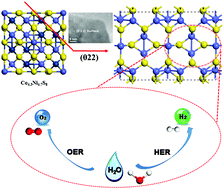
Developing an efficient non-noble bifunctional electrocatalyst for both the oxygen evolution reaction (OER) and the hydrogen evolution reaction (HER) in the same electrolyte is significant for lowering the cost of electrochemical water splitting. Herein, a phase-pure pentlandite Ni4.3Co4.7S8 bifunctional electrocatalyst was synthesized via a hydrothermal process using a commercial nickel foam as the nickel source. The active metallic nickel source and the chelating agent ethylenediamine play important roles in the formation of phase-pure pentlandite Ni4.3Co4.7S8 binary sulfide. Physicochemical characterizations, electrochemical measurements and density functional theory (DFT) computations illustrate that the material has an exposed high-indexed (022) surface with a biomimetic hydrogenase-like structure, and that the pentlandite phase has metallic characteristics, with next-nearest neighbor metal–metal bonds, as well as there being a high overlap of density of state (DOS) at the Fermi-level due to the synergistic effect between Ni and Co ions. In addition, there is an elevation of the d-state center (from −2.84 to −1.52 eV) with high occupation of the anti-bonding eg (dx2−y2 and dz2) d-orbitals. These properties endow the Ni4.3Co4.7S8 bifunctional electrocatalyst with higher catalytic activity for OER than RuO2, with comparative activity for HER to commercial Pt/C and with a low over-potential for all water splitting in an alkaline electrolyte. The studies here provide a novel strategy to synthesise phase-pure pentlandite nickel cobalt binary sulfides and boost their applications in electrochemical water splitting.


 Please wait while we load your content...
Please wait while we load your content...