Hierarchical branched platinum–copper tripods as highly active and stable catalysts†
Abstract
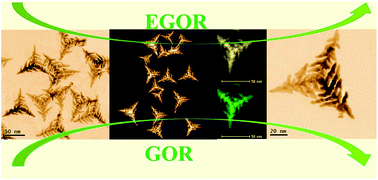
Designing and manipulating the structure of nanomaterials can efficiently tailor their catalytic properties, enabling the promotion of both their activity and stability. We herein report the shape-controlled synthesis of advanced Pt–Cu hierarchical tripod nanocrystals (HTNCs) by controlling the amount of KI and reaction time. The as-prepared nanocrystals (NCs) look like a typical tripod on the whole, consisting of similar branch structural units. In addition, the structure of the HTNCs could also be obtained with a narrow Pt/Cu feeding ratio. Owing to the unique HTNC structure and exposed high-index facets, as well as probable electronic effects between Cu and Pt, the as-obtained Pt–Cu HTNCs can exhibit greatly enhanced electrocatalytic activity toward ethylene glycol oxidation reaction (EGOR) and glycerol oxidation (GOR), which are 5.1 and 6.5 times higher in mass activity, as well as 5.6 and 7.3 times higher in specific activity relative to commercial Pt/C, showing that they are a class of promising electrocatalyst for fuel cells. This work presents huge opportunities for optimizing the electrocatalytic oxidation reaction by designing the structure of nanocatalysts.


 Please wait while we load your content...
Please wait while we load your content...