Sulfonate-ended carbosilane dendrimers with a flexible scaffold cause inactivation of HIV-1 virions and gp120 shedding†
Abstract
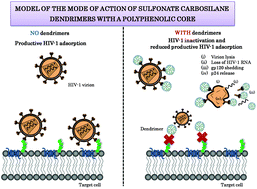
Infection with human immunodeficiency virus type 1 (HIV-1) continues to be a global public health issue, especially in low-resource countries. Sexual transmission is responsible for the majority of HIV-1 infections worldwide. Women are more susceptible to HIV-1 acquisition than men and represent nearly 50% of the HIV-infected population. Topical vaginal microbicides that act at the earlier stages of infection offer a prevention strategy to reduce the acquisition of HIV-1. Dendrimers are nano-sized, radially symmetric molecules with a well-defined and monodisperse structure consisting of tree-like arms or branches. We perform a TZM.bl cell line-based screening of two families of carbosilane dendrimers (6 nanocompounds: G1-S12P, G2-S24P, G3-S48P, G1-C12P, G2-C24P and G3-C48P) that we have previously synthesized, containing 12, 24 or 48 sulfonate (or carboxylate) end-groups and a polyphenolic core. This work shows that second- and third-generation sulfonate-ended carbosilane dendrimers with a polyphenolic core (G2-S24P and G3-S48P, respectively) display low cytotoxicity (CC50 > 300 μM) with virucidal anti-R5-HIV-1 activity (EC50 < 50 nM; therapeutic index >6000) causing irreversible HIV-1 inactivation (80–90%) by loss of HIV-1 RNA (40%), gp120 shedding (70–80%) and p24 capsid protein release (45–60%). Herein, we demonstrate that sulfonate end-groups and a flexible scaffold from carbosilane dendrimers strongly influence their properties acting as potent virucides.
- This article is part of the themed collection: 2018 Nanoscale HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...