Anomalous cation diffusion in salt-doped confined bilayer ice†
Abstract
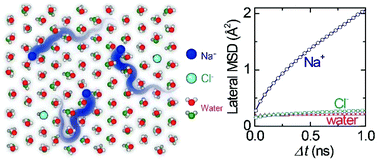
The diffusive dynamics of aqueous electrolyte solutions in nanoconfined spaces has attracted considerable attention due to their potential applications in desalination, biosensors and supercapacitors. Here we show by molecular dynamics simulations that lithium and sodium ions diffuse at a rate at least an order of magnitude higher than that of water molecules when the ions are trapped in an ice bilayer confined between two parallel plates. This novel picture is in sharp contrast to the prevailing view that the diffusion rate of ions is comparable to or even lower than that of water in both bulk and confined solutions. The predicted high ion mobility stems from frequent lateral hopping of ions along the coordination sites inside the hydrogen-bonding network connecting the two water layers of the ice bilayer. This anomalous diffusion should provide new insights into the physics of confined aqueous electrolytes.


 Please wait while we load your content...
Please wait while we load your content...