Unraveling aminophosphine redox mechanisms for glovebox-free InP quantum dot syntheses†
Abstract
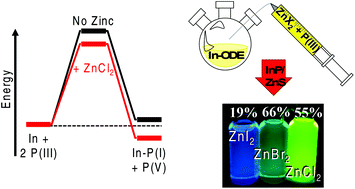
The synthesis of colloidal indium phosphide quantum dots (InP QDs) has always been plagued by difficulties arising from limited P3− sources. Being effectively restricted to the highly pyrophoric tris(trimethylsilyl) phosphine (TMS3P) creates complications for the average chemist and presents a significant risk for industrially scaled reactions. The adaptation of tris(dialkylamino) phosphines for these syntheses has garnered attention, as these new phosphines are much safer and can generate nanoparticles with competitive photoluminescence properties to those from (TMS)3P routes. Until now, the reaction mechanics of this precursor were elusive due to many experimental optimizations, such as the inclusion of a high concentration of zinc salts, being atypical of previous InP syntheses. Herein, we utilize density functional theory calculations to outline a logical reaction mechanism. The aminophosphine precursor is found to require activation by a zinc halide before undergoing a disproportionation reaction to self-reduce this P(III) material to a P(-III) source. We use this understanding to adapt this precursor for a two-pot nanoparticle synthesis in a noncoordinating solvent outside of glovebox conditions. This allowed us to generate spherical InP/ZnS nanoparticles possessing fluorescence quantum yields >55% and lifetimes as fast as 48 ns, with tunable emission according to varying zinc halide acidity. The development of high quality and efficient InP QDs with this safer aminophosphine in simple Schlenk environments will enable a broader range of researchers to synthesize these nontoxic materials for a variety of high-value applications.


 Please wait while we load your content...
Please wait while we load your content...