Synthesis of nickel germanide (Ge12Ni19) nanoparticles for durable hydrogen evolution reaction in acid solutions†
Abstract
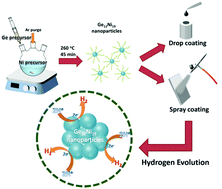
Desigining advanced materials as electrochemical catalysts for the hydrogen evolution reaction (HER) has caught great attention owing to the growing demand for clean and renewable energy. Nickel (Ni)-based compounds and alloys are promising non-noble-metal electrocatalysts due to their low cost and high activity. However, in most cases, Ni-based compounds and alloys have low durability in acid electrolyte, which limits their application in the electrolytic processes. In this study, monoclinic Ge12Ni19 nanoparticles were synthesized and exhibited high electrocatalytic activity and stability for the HER in acidic solution. Ge12Ni19 nanoparticles achieve an overpotential of 190 mV at cathodic current density of 10 mA cm−2 and a Tafel slope of 88.5 mV per decade in 0.50 M H2SO4 electrolyte. Moreover, the performance is maintained after a 10 000-cycle CV sweep (−0.3 to +0.1 V vs. RHE) or under a static overpotential of −0.7 V vs. RHE for 24 hours. The reported electrocatalytic performance of the Ge12Ni19 nanoparticles sufficiently proves the excellent endurance at lower required active overpotentials in acidic solution, enabling the broad applications of the Ni-based electrocatalysts. Finally, a large-area (5 cm2) electrocatalyst for HER was demonstrated for the first time. The great efficiency of the energy conversion performance sufficiently represented the potential of Ge12Ni19 nanoparticles as electrocatalysts in commercial fuel cells.


 Please wait while we load your content...
Please wait while we load your content...