Rapid reaction of MoS2 nanosheets with Pb2+ and Pb4+ ions in solution†
Abstract
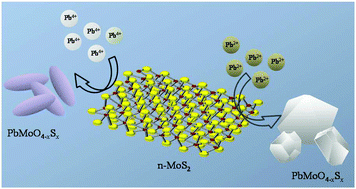
Understanding the chemical changes happening to nanostructures during a process is vital in selecting them for applications. Here, we investigated the difference in the reactivity of the bulk and nanoscale forms of molybdenum disulfide (MoS2) in solution with lead ions (Pb2+ and Pb4+) as probes, at room temperature. While the bulk form did not show any reactivity in the experimental timescale, the two-dimensional (2D) nanoscale form showed not only reactivity but also quite rapid kinetics that resulted in the formation of distinct products, principally PbMoO4 with anion substitution, in a few seconds. Depending on the charge state of the cation, and the pH of the reaction mixture, two different kinds of morphologies of the same reaction product were formed. Furthermore, we demonstrate that this unusual reactivity of the MoS2 nanosheets (NSs) was retained in its supported form and hence, such supported materials can be effective for the abstraction of toxic lead from water, with fast kinetics.


 Please wait while we load your content...
Please wait while we load your content...