Computational insight into the origin of unexpected contrast in chiral markers as revealed by STM†
Abstract
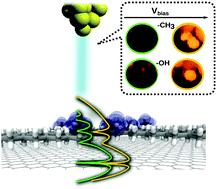
Internal substituents can serve the double purpose of generating stereogenic centers and (potentially) being identifiable with Scanning Tunneling Microscopy (STM) in 2D self-assembled molecular layers. We investigate computationally the origin of stark contrast variations in STM images of chirally substituted self-assembled organic films. STM images of alkyl derivatives with secondary –CH3 and –OH groups have been simulated. Density functional theory calculations reveal bias-dependent contrast reversals in the substituent regions: a lack of local density of states in the relevant energy regime results in ‘dark spots’ in the simulated STM images, which turn bright upon increasing the bias voltage.


 Please wait while we load your content...
Please wait while we load your content...