Facile synthesis of pyrite (FeS2/C) nanoparticles as an electrode material for non-aqueous hybrid electrochemical capacitors†
Abstract
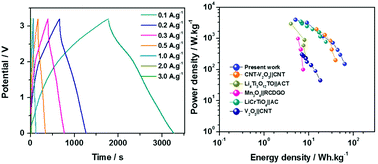
Pyrite (FeS2) is a promising electrode material for lithium ion batteries (LIBs) because of its high natural availability, low toxicity, cost-effectiveness, high theoretical capacity (894 mA h g−1) and high theoretical specific energy density (1270 W h kg−1, 4e−/FeS2). Nevertheless, the use of FeS2 in electrochemical capacitors was restricted due to fast capacity fading as a result of polysulfide (S/Sn2−) formation during the initial electrochemical cycling. In order to avoid the formation of polysulfides, we employed the strategy of utilizing an ether based electrolyte (1.0 M lithium bis(trifluoromethanesulfonyl)imide (LiTFSI)/diglyme (DGM)). Herein, we introduce FeS2/C as the Faradaic electrode for a non-aqueous hybrid electrochemical capacitor (NHEC) in combination with activated carbon (AC) as a non-Faradaic electrode, and 1.0 M LiTFSI/DGM as a non-aqueous electrolyte. Specifically, FeS2/C nanoparticles have been prepared via the sulfidation of a room temperature synthesized Fe-based MOF (metal organic framework) precursor. The fabricated FeS2/C∥AC NHEC, operating within the chosen voltage window of 0–3.2 V, delivered energy densities in the range of 63–9 W h kg−1 at power densities of 152–3240 W kg−1. Remarkable cycling stability with stable energy density retention for 2500 cycles at high power densities (729, 1186 and 3240 W kg−1) was observed.


 Please wait while we load your content...
Please wait while we load your content...