Total synthesis campaigns toward chlorophylls and related natural hydroporphyrins – diverse macrocycles, unrealized opportunities†
Abstract
Covering: up to 2018
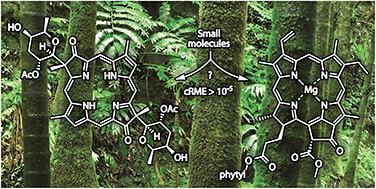
Chlorophylls, bacteriochlorophylls and related hydroporphyrins constitute invaluable natural products but have largely remained outside the scope of viable syntheses. The campaign toward chlorophyll a by Woodward and coworkers is a deservedly celebrated landmark in organic synthesis yet the route entailed 49 steps, relied on semisynthetic replenishment of advanced intermediates, and then pointed to (but did not implement) uncertain literature procedures for the final transformations. Indeed, the full synthesis at any scale of any (bacterio)chlorophylls – conversion of small-molecule starting materials to the product – has never been accomplished. Herein, the reported syntheses of (±)-bonellin dimethyl ester (0.93 mg) and tolyporphin A O,O-diacetate (0.38 mg), as well as the never-fully traversed route to chlorophyll a, have been evaluated in a quantitative manner. Bonellin and tolyporphin A are naturally occurring chlorin and bacteriochlorin macrocycles, respectively, that lack the characteristic fifth ring of (bacterio)chlorophylls. A practical assessment is provided by the cumulative reaction mass efficiency (cRME) of the entire synthetic process. The cRME for the route to chlorophyll a would be 4.3 × 10−9 (230 kg of all reactants and reagents in total would yield 1.0 mg of chlorophyll a), whereas that for (±)-bonellin dimethyl ester or tolyporphin A O,O-diacetate is approximately 6.4 × 10−4 or 3.6 × 10−5, respectively. Comparison of the three syntheses reveals insights for designing hydroporphyrin syntheses. Development of syntheses with cRME > 10−5 (if not 10−4), as required to obtain 10 mg quantities of hydroporphyrin for diverse physicochemical, biochemical and medicinal chemistry studies, necessitates significant further advances in tetrapyrrole chemistry.


 Please wait while we load your content...
Please wait while we load your content...
