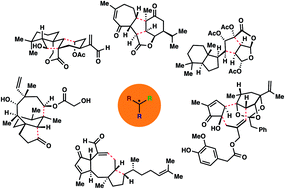
Total synthesis of complex terpenoids employing radical cascade processes
Abstract
Covering: 2011–2017
Radical cyclizations have a rich history in organic chemistry and have been particularly generous to the field of natural product synthesis. Owing to their ability to operate in highly congested molecular quarters, and with significant functional group compatibility, these transformations have enabled the synthesis of numerous polycyclic terpenoid natural products over the past several decades. Moreover, when programmed accordingly into a synthetic plan, radical cascade processes can be used to rapidly assemble molecular complexity, much in the same way nature rapidly constructs terpene frameworks through cationic cyclization pathways. This review highlights recent total syntheses of complex terpenoids (from 2011–2017) employing C–C bond-forming radical cascade sequences.


 Please wait while we load your content...
Please wait while we load your content...