Expanding the roles for 2-oxoglutarate-dependent oxygenases in plant metabolism
Abstract
Covering: up to 2018
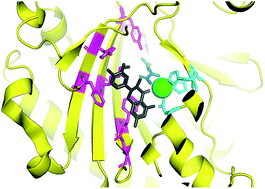
2-Oxoglutarate-dependent oxygenases (2ODOs) comprise a large enzyme superfamily in plant genomes, second in size only to the cytochromes P450 monooxygenase (CYP) superfamily. 2ODOs participate in both primary and specialized plant pathways, and their occurrence across all life kingdoms points to an ancient origin. Phylogenetic evidence supports substantial expansion and diversification of 2ODOs following the split from the common ancestor of land plants. More conserved roles for these enzymes include oxidation within hormone metabolism, such as the recently described capacity of Dioxygenase for Auxin Oxidation (DAO) for governing auxin homeostasis. Conserved structural features among 2ODOs has provided a basis for continued investigation into their mechanisms, and recent structural work is expected to illuminate intriguing reactions such as that of 1-aminocyclopropane-1-carboxylic acid oxidase (ACCO). Phylogenetic radiation among this superfamily combined with neo- and subfunctionalization has enabled recruitment to highly specialized pathways, including those yielding medicines, flavours, dyes, poisons, and compounds important for plant-environment interactions. Catalytic versatility of 2ODOs in plants and across broader taxa continues to inspire biochemists tasked with the discovery of new enzymes. This highlight article summarizes recent reports up to 2018 of 2ODOs within plant metabolism. Furthermore, the respective contributions of 2ODOs and other oxidases to natural product biosynthesis are discussed as a framework for continued discovery.
- This article is part of the themed collection: Metalloenzymes in Natural Product Biosynthetic Pathways


 Please wait while we load your content...
Please wait while we load your content...