TiO2 nanoparticles coated with deep eutectic solvents: characterization and effect on photodegradation of organic dyes†
Abstract
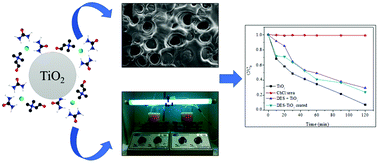
Deep eutectic solvents (DES) have been used as sustainable media for the synthesis of more efficient nanoparticle-based catalysts. However, the effects of DES deposition on the catalytic activity of metal oxide nanoparticles like TiO2 are unknown. Thus, the goal of this study was to facilitate the deposition of DES onto TiO2 surfaces and investigate how this coating modulates the catalytic activity of TiO2 in the photodecomposition of organic dyes. Results for the thermal and spectroscopic properties of the TiO2 coated with DES in relation to pure materials indicated interactions between these two materials, confirming the deposition of DES onto the TiO2 surface. Different choline chloride : HBD molar ratios had little impact on the thermal stability, phase transitions, crystallization and amorphization of the mixture. In general, the catalytic activity of TiO2 coated with DES was reduced. However, TiO2 covered with ChCl : malonic acid (1 : 2) showed the best catalytic activity and it was better as greater the amount of DES used in the surface deposition.


 Please wait while we load your content...
Please wait while we load your content...