Synergic effect on oxygen reduction reaction of strapped iron porphyrins polymerized around carbon nanotubes†
Abstract
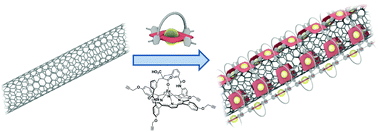
In the context of the development of new bio-inspired catalysts, MN4 complexes exhibit a great potential for small molecule activation. In particular, metallated porphyrins and phthalocyanines combined with carbon nanotubes have been tested for the oxygen reduction reaction in electrocatalytic systems, and these nanotube/MN4 hybrids have demonstrated promising properties. Here, a series of hybrid materials made of multi-walled carbon nanotubes (MWNTs) coated with strapped porphyrins have been fabricated. Iron porphyrin derivatives were polymerized around the nanotubes via Hay coupling and the resulting materials were fully characterized. Two porphyrins were probed; both were strapped with the same skeleton and they differed only in the presence or absence of overhung carboxylic acids. In the porphyrin, the carboxylic acid group could possibly act as a proton relay between the medium and the catalyst. Although the presence of the carboxylic acid groups (acting as intramolecular proton relays) did not exhibit a significant influence on the catalytic properties, the combination of both components – the MWNTs and porphyrin – led to a better catalytic activity than those of the nanotubes or the porphyrins taken separately. The synergic affect is due to the MWNTs which ensure the availability of electrons to the porphyrin catalysts and allow the ORR to occur via the 4-electron pathway, avoiding the production of hydrogen peroxide.


 Please wait while we load your content...
Please wait while we load your content...
