Fabrication of magnetic g-C3N4 for effectively enhanced tetracycline degradation with RGO as mediator†
Abstract
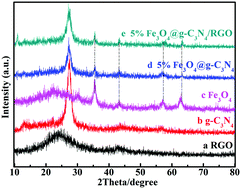
In the present work, the ternary hybrid photocatalyst Fe3O4@g-C3N4/RGO is developed by a facile hydrothermal method, which shows a satisfactory degradation rate (90%) and stability in degrading tetracycline. The improvement in the photocatalytic activity of the ternary hybrid photocatalyst is attributed to the Fe3O4 nanobelt and RGO. As a center of oxidation–reduction, the Fe3O4 nanobelt can utilize electrons effectively. RGO with good conductivity is an outstanding electron transporter, which inhibits the recombination of electron–hole pairs. Moreover, the results of the ESR and the radical capture experiment prove that h+ and ˙O2− are the major active species in the tetracycline removal process. At the same time, the degradation intermediates of tetracycline and the photocatalytic mechanism is discussed in this paper in depth. The novel design concept is expected to be used in practical applications.


 Please wait while we load your content...
Please wait while we load your content...