Diphenylamino-substituted tristyryl vs. triphenyl isocyanurates: improved conjugation has minimal impact on two-photon absorption†
Abstract
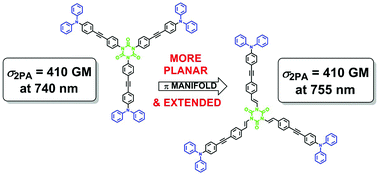
This contribution reports the synthesis of C87H60N6O3 (3-NPh2), the tristyryl analogue of the triphenylisocyanurate C81H54N6O3 (2-NPh2) which was previously demonstrated to be an active two-photon absorber. Contrary to expectation, planarization and extension of the central π-system does not lead to a marked improvement of the two-photon absorption cross-section.


 Please wait while we load your content...
Please wait while we load your content...
