Antioxidant properties and free radical scavenging mechanisms of cyclocurcumin
Abstract
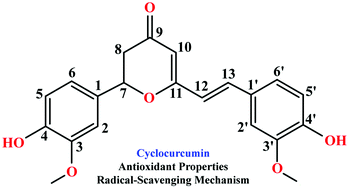
Recently, cyclocurcumin, a seldom studied minor component of curcuminoids, has been found to exhibit abundant bioactivities. To explore the antioxidant properties and free radical scavenging mechanisms of neutral and deprotonated cyclocurcumins, a theoretical study, based on density functional theory using the exchange–correlation M052X functional in connection with the 6-311++G(d,p) basis set, was employed. Their thermodynamic parameters (bond dissociation enthalpy, electron transfer enthalpy, ionization potential, proton affinity and proton dissociation enthalpy) were computed in order to establish what among the hydrogen-atom transfer, single electron transfer-proton transfer and sequential proton loss-electron transfer reaction mechanisms is preferentially followed by cyclocurcumin to inactivate ˙OH and ˙OOH free radicals in water and chlorobenzene. The results show that cyclocurcumin has strong activity as a scavenger of ˙OH and ˙OOH free radicals preferentially by its 4′-OH phenolic radical via a hydrogen-atom transfer mechanism in water and a physiological environment. The solvent effects are not significant for the preferred mechanism.


 Please wait while we load your content...
Please wait while we load your content...