Adsorptive removal of Cd2+ from aqueous solutions by a highly stable covalent triazine-based framework
Abstract
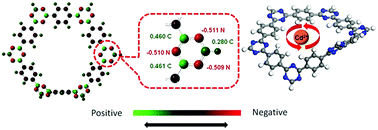
Porous crystalline materials such as covalent organic frameworks (COFs) have gained tremendous popularity in multidisciplinary areas of science and technology. In this study, for the first time, we report a covalent triazine-based framework (CTF-1) as an efficient adsorbent for the removal of Cd2+ from aqueous solutions. CTF-1 offered excellent stability under extreme conditions for the effective removal of cadmium cations (Cd2+) from aqueous solutions. CTF-1 was first synthesized through an ionothermal method and then characterized by XRD, SEM, TEM and BET surface area measurements to confirm its highly crystalline and microporous nature. Batch adsorption experiments were systematically conducted under a wide range of pH, metal ions concentration, adsorbent dosage and contact time to investigate kinetics, thermodynamics and adsorption properties of CTF-1 towards Cd2+ ions removal. The pseudo-second-order model showed good fitting to experimental data, whereas Langmuir and D–R isotherms confirmed the chemical nature of the adsorption. Similarly, thermodynamic parameters indicated the adsorption to be spontaneous and endothermic. Furthermore, our simulation results showed that CTF-1 possess uniform distribution of negative charge along the framework, making it an ideal candidate for the adsorption of cations together with the high stability in both acidic and basic pH. The strategies adopted in this study will open a new avenue to design novel nanoporous functional materials for next generation adsorbents.


 Please wait while we load your content...
Please wait while we load your content...