Preparation of Y2SiO5:Pr3+,Li and Na2NbxTa2−xO6/(Au/RGO) composites and investigation into visible-light driven photocatalytic hydrogen production
Abstract
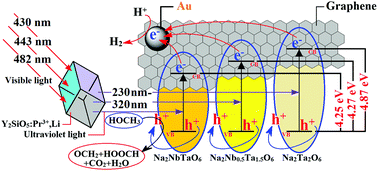
A visible-light-driven photocatalytic hydrogen production system (Y2SiO5:Pr3+,Li/Na2NbxTa2−xO6/(Au/RGO)), which is composed of an up-conversion luminescence agent (Y2SiO5:Pr3+,Li), Nb(V)-substituted Na2Ta2O6 (Na2NbxTa2−xO6) and a combined co-catalyst (Au/RGO), is designed and the photocatalytic hydrogen production activity is studied using methanol as the sacrificial agent in aqueous solution. In the employed composite nanoparticles, the Y2SiO5:Pr3+,Li/Na2Nb0.5Ta1.5O6/(Au/RGO) system with 0.4 : 1.0 mass ratio of Y2SiO5:Pr3+,Li and Na2Nb0.5Ta1.5O6 affords the highest amount of hydrogen production under visible-light irradiation, which is 2.3 times higher than that of Na2Nb0.5Ta1.5O6/(Au/RGO). The high photocatalytic hydrogen production activity is attributed to the spectrographic matching between Y2SiO5:Pr3+,Li and Na2Nb0.5Ta1.5O6/(Au/RGO). That is, the ultraviolet-light (230–320 nm) from the up-conversion emission of Y2SiO5:Pr3+,Li can effectively activate Na2Nb0.5Ta1.5O6/(Au/RGO). The presence of Nb can lead to the adjustment of the band-gap of Na2Ta2O6 to broaden the light response range. In addition, Au/RGO can quickly separate the photo-generated electrons and holes and further enhance the photocatalytic hydrogen production activity.


 Please wait while we load your content...
Please wait while we load your content...