Facile selective synthesis of 2-methyl-5-amino-1,2,4-oxadiazolium bromides as further targets for nucleophilic additions†
Abstract
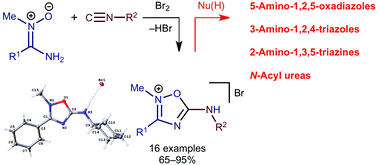
The reaction of aminonitrones R1C(NH2) = N+(Me)O− (R1 = Alk, Ar) with isocyanides R2NC (R2 = Alk, Ar; 1.2 equiv.) and Br2 (1 equiv.) conducted in CHCl3 (RT, 5 min) gives 2-methyl-5-amino-1,2,4-oxadiazolium bromides in good to excellent yields (65–95%; 16 examples). These species are highly electrophilically activated and 5-cyclohexylamino-2-methyl-3-phenyl-1,2,4-oxadiazolium bromide, taken as a model compound for the reactivity study, reacts rapidly under mild conditions with hydroxylamine, hydrazine, or benzamidine, to give 5-cyclohexylamino-3-phenyl-1,2,4-oxadiazole (88%), 5-cyclohexylamino-3-phenyl-1,2,4-triazole (95%), and 2-cyclohexylamino-4,6-diphenyl-1,3,5-triazine (64%), respectively. Treatment of the oxadiazolium salt with excess water provides N-benzoyl-N′-cyclohexylurea (95%).


 Please wait while we load your content...
Please wait while we load your content...