A comparative study of the effects of terminal aromatic moieties in spirobifluorene core-based diketopyrrolopyrrole non-fullerene acceptors†
Abstract
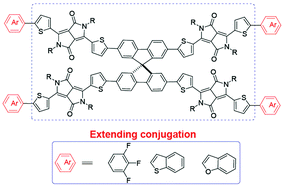
This work focuses on the development of diketopyrrolopyrrole (DPP)-based small molecular nonfullerene acceptors to pair traditional and low-cost poly(3-hexylthiophene) (P3HT) in bulk heterojunction (BHJ) organic solar cells. Different end capped groups, 1,2,3-trifluorobenzene and fused-ring moieties (benzo[b]thiophene and benzo[b]furan), were separately introduced into promising DPP-based nonfullerenes SF-DPP-EH, and three new spiro-DPPs SF-(DPP3F)4, SF-(DPPBT)4 and SF-(DPPBF)4) were obtained. These terminal groups largely extend the conjugation in SF-DPP-EH with an obvious red-shifted absorption (25–43 nm). Of the three new spiro-DPPs, SF-(DPP3F)4 showed the best solubility in common solvents and gave a power conversion efficiency of 1.67% when blended with P3HT. The charge-transfer state (ECT) at the donor–acceptor interface was directly probed through measuring the subbandgap external quantum efficiency (EQE) to estimate the energy loss in spiro-DPP based devices.


 Please wait while we load your content...
Please wait while we load your content...