Copper(i)/succinic acid cooperatively catalyzed one-pot synthesis of organoselenium-propargylamines via A3-coupling†
Abstract
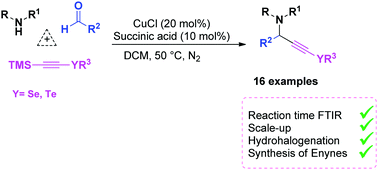
Selenium propargylamines were synthesized via an A3-coupling approach using piperidine, p-methoxybenzaldehyde, and trimethylsilyl selenium–acetylene, catalyzed by copper(I) chloride and succinic acid as an additive, in good to excellent yields. This method is advantageous in that desilylation of the trimethylsilyl selenium–acetylene occurs in situ. The reaction time was monitored by FTIR studies and a probable mechanism is described. Scale-up was also performed in satisfactory yield. These selenium propargylamines could be hydrohalogenated to synthesize halovinyl selenides. The stereochemistry was determined by treatment with n-butyllithium and the coupling constant (J) values in 1H NMR spectra. The vinyl selenides were employed in Sonogashira cross-coupling reactions to produce the corresponding enynes, with yields ranging from moderate to good.


 Please wait while we load your content...
Please wait while we load your content...