Theoretical characterization of supramolecular complexes formed by fullerenes and dimeric porphyrins
Abstract
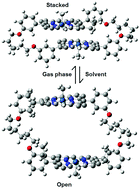
We have employed the M06-2X, PBE-D3BJ and B3LYP-D3BJ methods to study the hosting of fullerenes by 15 single, dimeric and trimeric porphyrins. We found a poor correlation between the experimental association constants and the calculated free energy changes in solution. Surprisingly, the correlation between ΔE and 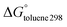 was also unsatisfactory. The problem could be traced back to the fact that dimeric porphyrins adopt stacked structures in the gas phase, while they present open structures in the supramolecular complex with C60. The inclusion of solvent effects reduced the relative energies between the stacked and open forms of the dimeric porphyrins. Thus, flexible dimeric porphyrins seem to be more efficient than buckybowls in overcoming non bonded interactions, which force hosts to adopt folded structures. For this reason they display larger association constants towards fullerenes. On the basis of these results, we found that a qualitative assessment of the strength of fullerene/porphyrin complexes was possible when diabatic interactions energies were considered, i.e. gas phase binding energies computed using the structures that the receptor and guest present in the supramolecular complex.
was also unsatisfactory. The problem could be traced back to the fact that dimeric porphyrins adopt stacked structures in the gas phase, while they present open structures in the supramolecular complex with C60. The inclusion of solvent effects reduced the relative energies between the stacked and open forms of the dimeric porphyrins. Thus, flexible dimeric porphyrins seem to be more efficient than buckybowls in overcoming non bonded interactions, which force hosts to adopt folded structures. For this reason they display larger association constants towards fullerenes. On the basis of these results, we found that a qualitative assessment of the strength of fullerene/porphyrin complexes was possible when diabatic interactions energies were considered, i.e. gas phase binding energies computed using the structures that the receptor and guest present in the supramolecular complex.


 Please wait while we load your content...
Please wait while we load your content...