A facile 2H-chromene dimerization through an ortho-quinone methide intermediate catalyzed by a sulfonyl derived MIL-101 MOF†
Abstract
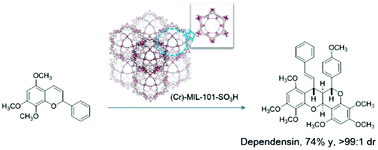
A MIL-101–SO3H MOF was synthesized using commercially available materials. The as-synthesized MIL-101–SO3H was characterized by SEM, XRD, FTIR, BET and TGA. An efficient and diastereoselective homo-dimerization of 2H-chromenes catalysis was achieved using the sulfonyl derived MIL-101 MOF as a catalyst. A benzopyranobenzopyran polycyclic structure was generated in high yield and diastereoselectivity under mild catalytic reaction conditions. Furthermore, a regio-isomer was observed when 2H-chromene bears different substitution groups, furnishing chromeno[2,3-b]chromene dimer in high yield and good diastereoselectivity. A mechanism is proposed with a tandem rearrangement/hetero-Diels–Alder reaction sequence which is supported by evidence. In addition, the acid MIL-101 MOF catalyst can be recycled ten times without compromising the yield and selectivity.


 Please wait while we load your content...
Please wait while we load your content...